We want to contribute to the eradication of human malaria by modifying mosquito populations to prevent malaria transmission in direct partnership and collaboration with local scientists, public health officials, government officials, and communities in an ethical and transparent manner.

A NEW SOLUTION:
ELIMINATING MALARIA NOT MOSQUITOES
UCMI is contributing to malaria eradication by using a genetic technology to modify target Anopheles mosquito populations to prevent malaria transmission. This is called “population modification” (also known as population replacement). It eliminates the ability of the mosquito to transmit malaria; it does not eliminate the mosquitoes.
POPULATION MODIFICATION
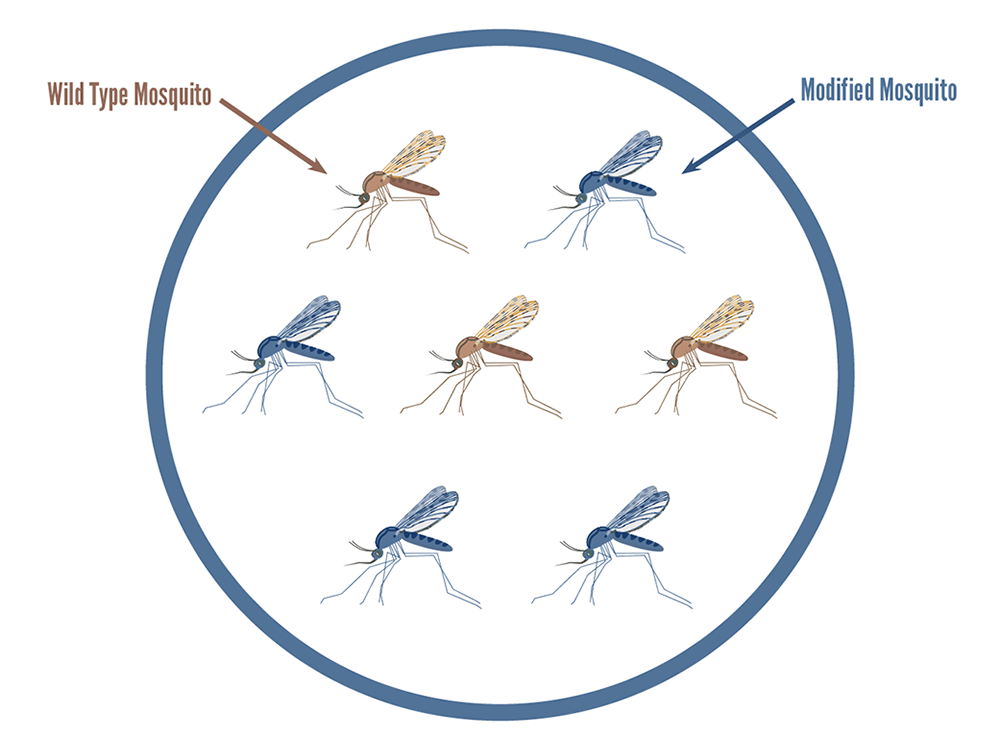
A genetic technology that alters natural mosquito populations to prevent them from transmitting malaria. This is achieved by coupling beneficial genes that block the parasite with a “gene drive” that can spread the beneficial genes in a mosquito population. This approach is also known as “population replacement” or “population alteration.”
BENEFICIAL GENES
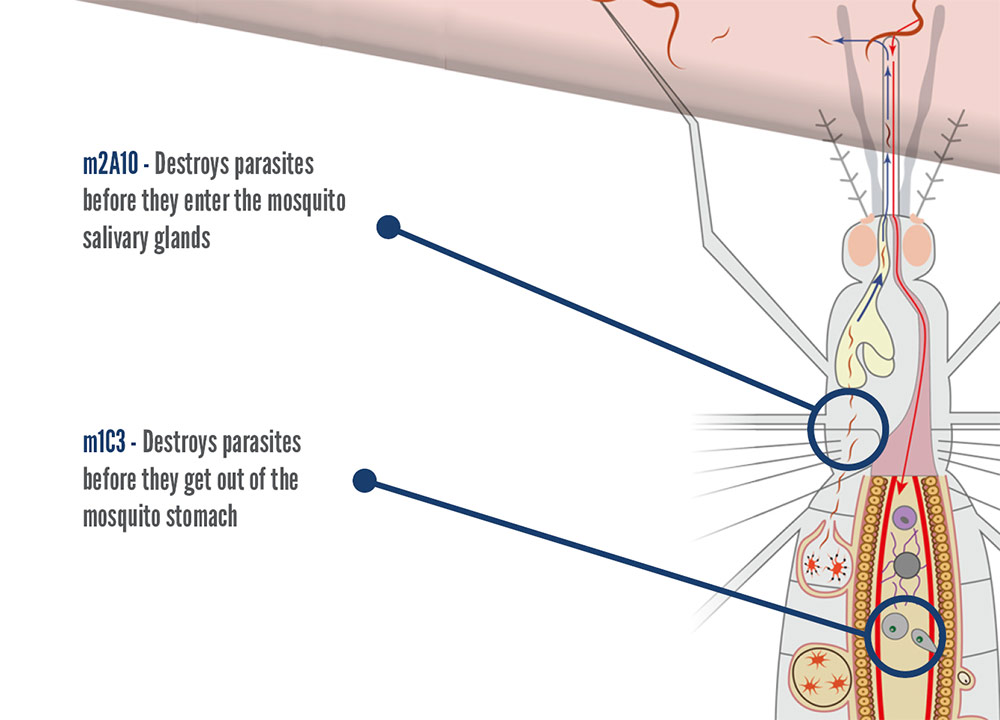
Modified mosquitoes have at least two beneficial genes designed specifically to block malaria parasite development in the modified mosquito. Beneficial genes are synthetic genes that are created in the UCMI laboratory.
GENE DRIVE
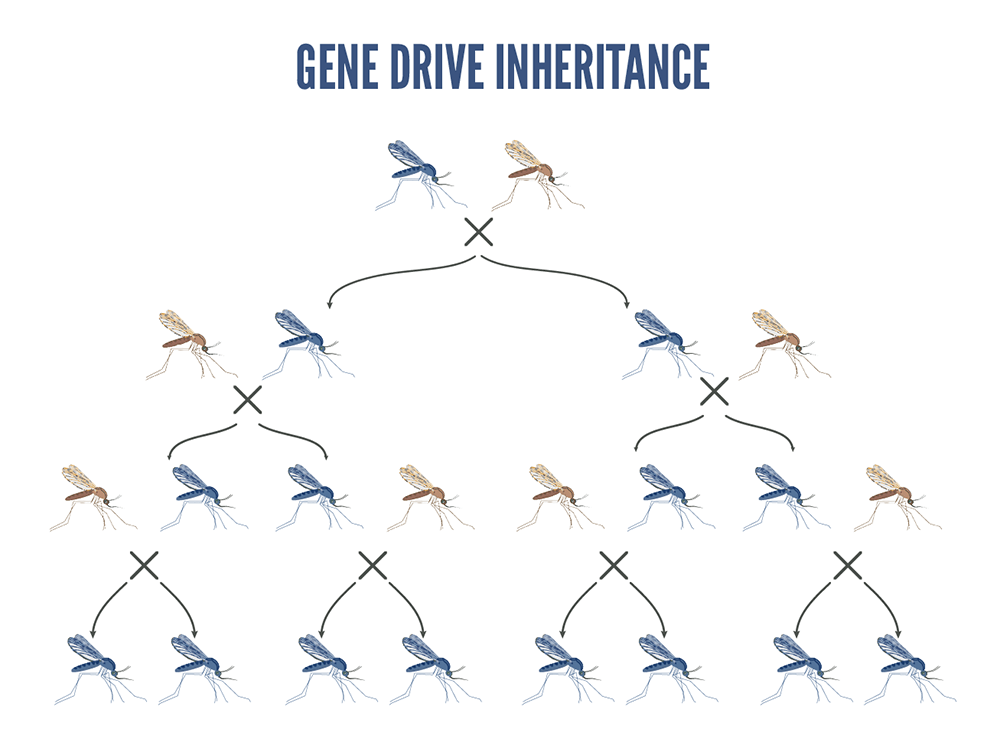
Beneficial genes are coupled with a gene drive system so that they become integrated into the mosquito DNA. Gene drive is a way to spread beneficial genes through mosquito populations at rates much higher than usual. Ordinarily, a gene is inherited from a parent by one-half (50%) of its offspring. Mosquito gene-drive technologies result in close to 99% of the progeny having the desired gene.
APPLYING THE TECHNOLOGY
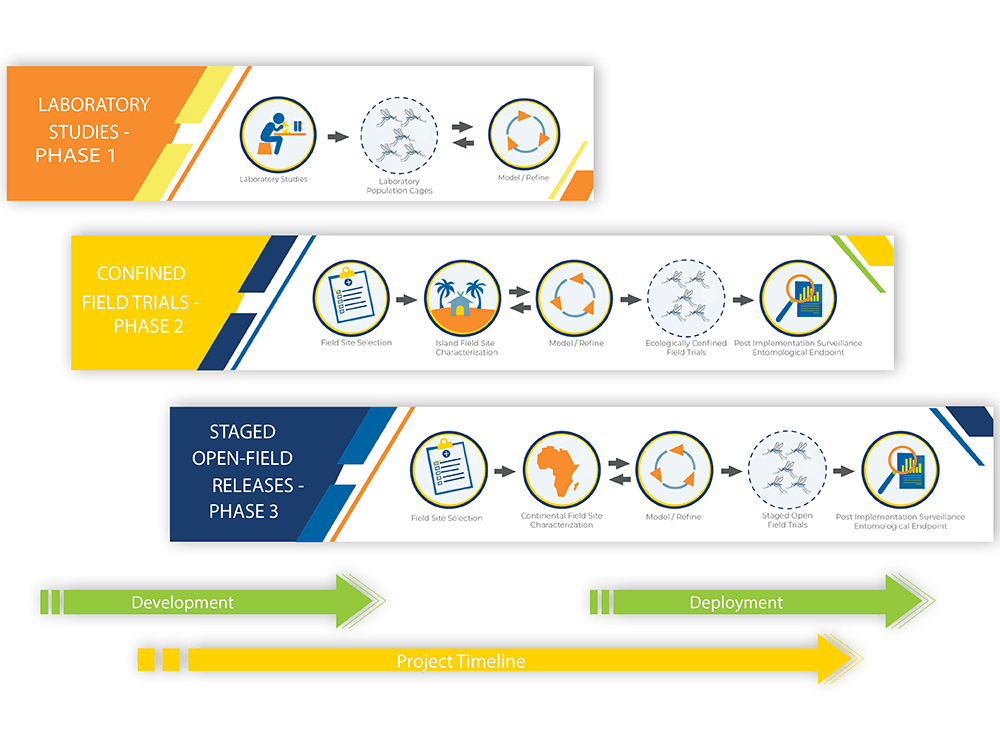
UCMI uses World Health Organization guidelines for testing genetically modified organisms. This means that before any mosquito with beneficial genes is released, there are phases of testing and data collection that must be done in partnership with the country that would like to consider this malaria control strategy.

Phase 1 includes the discovery and development of beneficial genes that prevent malaria parasite development without impacting the normal biology and function of the mosquito. Phase 1 also includes laboratory testing of the modified mosquito to ensure that the beneficial genes work and that the mosquitoes are able to resist parasite infection. This work has taken many years, and we have nearly completed laboratory testing with great success.
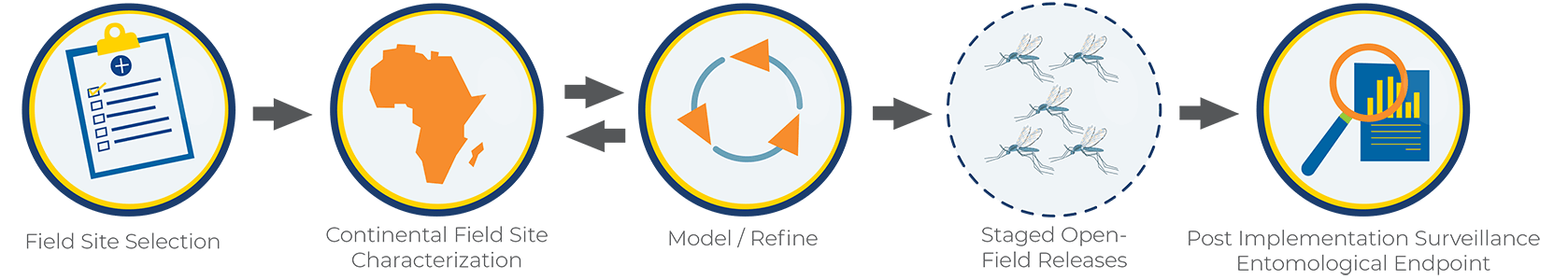
We are now at the beginning of Phase 2. A Phase 2 field trial involves several important steps:
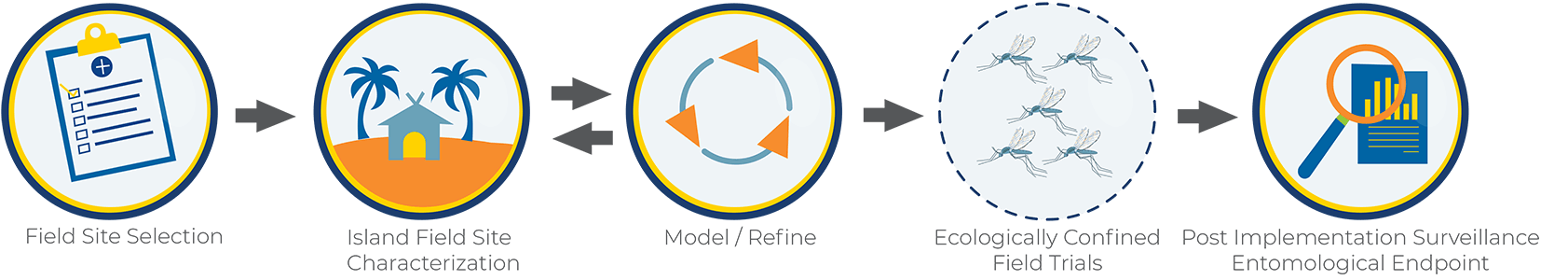
Field site selection: Suitable field sites should be isolated with limited movement of mosquitoes in and out (this is why is referred to as “confined”). We believe that oceanic island sites provide the best environment for a confined field trial.
Island field site characterization: Baseline data collection for 18-24 months which includes mosquito collections and analysis from many different places across the selected field site in collaboration with scientists and leaders at the field site. It also includes collaborative and ongoing Community and Stakeholder Engagement. This is the step of Phase 2 where we are currently.
Modeling and Refinement: Models are created using the baseline data that is collected at the field site to help inform best practices for a field trial.
Ecologically confined field trials: An eventual release of modified mosquitoes.
Post-implementation Surveillance: Evaluation and monitoring for several years after the modified mosquitoes are released.